Solved Problem on Magnetic Force
advertisement
In the region of the figure, we have a uniform magnetic field
\( \vec{B} \).
Five particles are thrown into this field at point O, all with initial velocity
v0. The particles are a proton, a neutral sodium atom, an electron, a deuteron, and a
negative fluorine ion. Characterize the trajectories described by the particles. Data: the deuteron is a
particle made up of a proton and a neutron; the mass of the fluorine ion is greater than that of the
electron and has the same charge.
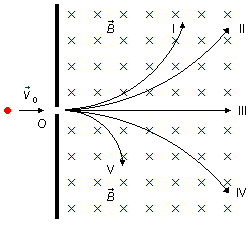
Problem data:
- As the deuteron consists of a proton (positive charge, qp > 0) and a neutron (no charge, qn = 0), the charge of the deuteron is positive: qd > 0;
- The mass of the fluorine ion is greater than that of the electron: mF > me;
- Fluorine ion charge: qF < 0;
- Electron charge: qe < 0.
When the particle enters the magnetic field region, the magnetic force begins to act on it. To determine the direction of this force, we will apply the left-hand rule to a particle crossing the magnetic field region.
The magnitude of the magnetic force is given by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{F=qvB\sin \theta} \tag{I}
\end{gather}
\]
where sin θ is the angle between the magnetic field vector
\( \vec{B} \)
and the velocity
\( {\vec{v}}_{0} \).
From the diagram of the problem, we see that the direction of the velocity of the particles is perpendicular
to the direction of the magnetic field (they make an angle of 90°), as sin 90° = 1, the force reduces to
\[
\begin{gather}
F=qvB \tag{II}
\end{gather}
\]
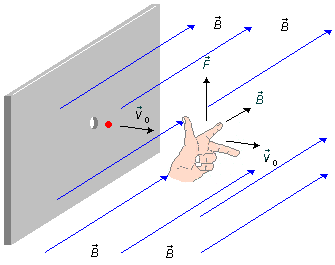
Figure 1 shows the problem in perspective. According to the diagram given in the problem, the magnetic field has a direction perpendicular to the sheet, the velocity has a horizontal direction from left to right
To apply the left-hand rule, we place the index finger in the direction of the electric field vector and the middle finger in the direction of velocity, so the thumb gives us the direction of the magnetic force, which will be vertical and upwards.
This force is valid for particles with a positive electric charge, q > 0, and for the negative particles, q < 0, just invert the direction of the force, the force will be vertical and pointing in the downwards direction. Particles with no electrical charge do not feel the action of the force.
So from the discussion above, we immediately have that trajectory (III) is of the neutral sodium atom that passes straight through the field without deviation.
The other particles undergo deviations with a varying radius of curvature in their trajectories, the following expression
\[
\begin{gather}
\bbox[#99CCFF,10px]
{R=\frac{mv}{qB} \tag{III}}
\end{gather}
\]
gives us the radius of the trajectory of the particle as a function of mass m, the velocity
v, the charge q, and the magnitude of the magnetic field B, analyzing this expression
for the various particles, we can identify their trajectories.From the discussion on the right-hand rule, we know that particles (I) and (II) have a positive electric charge, which in the problem are the proton and the deuteron, writing the expression (III)
\[
\begin{gather}
R_{p}=\frac{m_{p}v_{0}}{q_{p}B} \tag{IV}
\end{gather}
\]
\[
\begin{gather}
R_{d}=\frac{m_{d}v_{0}}{q_{d}B} \tag{V}
\end{gather}
\]
As the electric charges of the proton and the deuteron are equal and positive,
qp = qd > 0, and since the mass of the deuteron is greater than the
mass of the proton, md > mp, we have that the numerator of the
expression (V) is greater than the numerator of the expression (IV), then we conclude that
Rd > Rp, so the trajectory (I), smaller radius, is of the proton and
the trajectory (II), greater radius, is of the deuteron.Particles (IV) and (V) deviate downwards, they have negative charges, writing the expression (III) for the electron and for the fluorine ion
\[
\begin{gather}
R_{e}=\frac{m_{e}v_{0}}{q_{e}B} \tag{VI}
\end{gather}
\]
\[
\begin{gather}
R_{F}=\frac{m_{F}v_{0}}{q_{F}B} \tag{VII}
\end{gather}
\]
Their charges are equal and negative, qe = qF < 0, and the mass of
the fluorine ion is greater than that of the electron, so the numerator of expression (VII) is greater than
the numerator of expression (VI), and the radius of the trajectory of the fluorine ion will be greater than
the radius of the trajectory of the electron, RF > Re, the trajectory
(IV) will be of the negative fluorine ion and the trajectory (V) of the electron.
Summarizing:
- Trajectory (I) = proton;
- Trajectory (II) = deuteron;
- Trajectory (III) = neutral sodium atom;
- Trajectory (IV) = negative fluorine ion;
- Trajectory (V) = electron.
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .