Solved Problem on Thermodynamics
advertisement
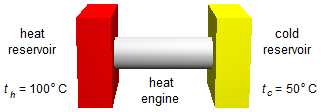
A heat engine works between a high-temperature reservoir at 100 °C and a lower-temperature reservoir at 50 °C, calculate:
a) The thermal efficiency of this machine;
b) The work done by the engine when receiving 10000 kcal of the high-temperature reservoir. Assume 1 cal = 4.2 J.
Problem data:
- Temperature of the hot reservoir: th = 100 °C;
- Temperature of the cold reservoir: tc = 50 °C.
Solution
First, we must convert temperatures given in degrees Celsius (°C) to kelvins (K) and energy (heat) given in item (b) in kilocalories (kcal) to joules (J), used in the International System of Units (S.I.)
\[
\begin{gather}
T_{h}=t_{q}+273=100+273=373\;\text{K}\\[10pt]
T_{c}=t_{f}+273=50+273=323\;\text{K}\\[10pt]
Q=10000\;\text{kcal}=10000\times 10^{3}\;\cancel{\text{cal}}\times \frac{4.2\;\text{J}}{1\;\cancel{\text{cal}}}=1\times 10^{7}\times 4.2\;\text{J}=4.2\times 10^{7}\;\text{J}
\end{gather}
\]
a) The thermal efficiency is given b
\[ \bbox[#99CCFF,10px]
{\eta =\frac{T_{h}-T_{c}}{T_{h}}}
\]
\[
\begin{gather}
\eta =\frac{373-323}{373}\\
\eta =\frac{50}{373}\\
\eta\simeq 0.13=\frac{13}{100}
\end{gather}
\]
\[ \bbox[#FFCCCC,10px]
{\eta =13\;\text{%}}
\]
b) Using the expression of the thermal efficiency as a function of work done W and the energy
received Q
\[ \bbox[#99CCFF,10px]
{\eta =\frac{W}{Q}}
\]
\[
\begin{gather}
W=\eta Q\\
W=0.13\times 4.2\times 10^{7}\\
W=5460000
\end{gather}
\]
\[ \bbox[#FFCCCC,10px]
{W=5.46\times 10^{6}\;\text{J}}
\]
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .