The hydrogen atom is made up of one proton and one electron. According to Bohr's atomic model, the electron
follows a circular trajectory, with the proton in the center.
Data:
electron mass:
\( 9.1\times 10^{-31}\;\mathrm{kg} \) ;
electron speed:
\( 2.2\times 10^6\;\mathrm{m/s} \) ;
proton charge:
\( 1.6\times 10^{-19}\;\mathrm C \) ;
electron charge:
\( -1.6\times 10^{-19}\;\mathrm C \) .
Determine the radius of the orbit of an electron for the atom in a vacuum.
Problem data:
- Electron mass: \( m=9.1\times 10^{-31}\;\mathrm{kg} \) ;
- Velocity of the electron: \( v=2.2\times 10^6\;\mathrm{m/s} \) ;
- Charge of the proton: \( q_p=1.6\times 10^{-19}\;\mathrm C \) ;
- Charge of the electron: \( q_e=-1.6\times 10^{-19}\;\mathrm C \) ;
- Coulomb Constant in a vacuum: \( k_e=9\times 10^9\;\mathrm{\frac{N.m^2}{C^2}} \) .
Problem diagram:
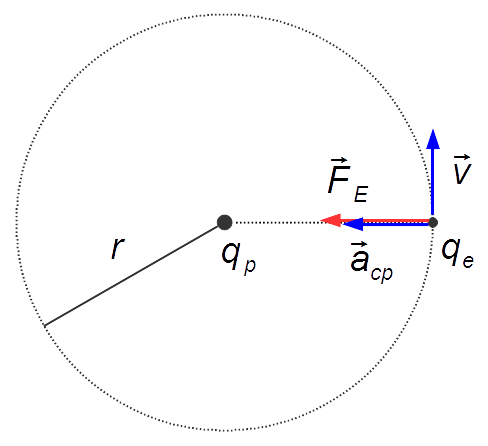
Solution:
Applying Coulomb's Law, the magnitude of the electric force is given by
The electron is in Uniform Circular Motion with constant speed, and a centripetal force acts on it, writing Newton's Second Law for a body in a circular motion
the centripetal acceleration is given by
substituting equation (III) into equation (I)
The proton and the electron have charges with opposite signs. The electric force between them is attractive, as this force coincides with the resultant centripetal force, equating equations (I) and (IV)

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .