Solved Problem on Thermodynamics
advertisement
The cylinder of a steam engine receives 2300 kcal per unit of time, of this total 2070 kcal are lost to the environment. What is the thermal efficiency of this engine?
Problem data:
- Heat received: Q1 = 2300 kcal;
- Heat lost: Q2 = 2070 kcal.
A quantity of heat Q1 is introduced into the cylinder, doing a work W pushing the piston, a quantity of heat Q2, unused at work, is expelled as steam (Figure 1).
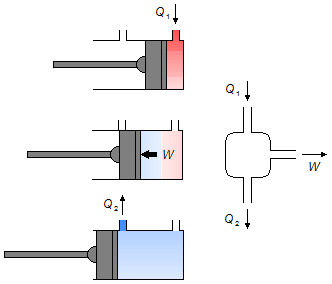
Solution
The thermal efficiency is given by
\[ \bbox[#99CCFF,10px]
{\eta =\frac{W}{Q_{1}}=\frac{Q_{1}-Q_{2}}{Q_{1}}}
\]
\[
\begin{gather}
\eta =\frac{2300-2070}{2300}\\
\eta=\frac{230}{2300}\\
\eta =0.1=\frac{10}{100}
\end{gather}
\]
\[ \bbox[#FFCCCC,10px]
{\eta =10\;\text{%}}
\]
Note: The values are given in kilocalories (kcal), but it is not necessary to consider this
factor
\( \text{kilo}=1000=10^{3} \),
since this value cancels out when dividing the calculation of efficiency.
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .